Chemistry is life. Or is life chemistry? Let’s just go with yes on both counts. Whether you can already recite the periodic table by atomic weight or you were expecting a list about Ryan Gosling and Rachel McAdams in The Notebook (get it? they had great chemistry!), there’s a lot to learn about this fascinating branch of science. Here are 46 of the most interesting facts about chemistry and its role in our lives.
Chemistry Facts
46. Pencil Body
Your body, yes yours, has enough graphite inside of it to produce roughly 9,000 pencils. See, you are special after all.
45. Airbag Safety
The airbag in cars can save your life, but they are actually made of a highly toxic substance called sodium azide. The airbag is full of these salts and they release once triggered by the vehicle to increase their temperature in order to burst the bag out of its compact place. Don’t worry though, as, after this fraction of a second increase in temperature, they transpose into nitrogen gas, which is what actually causes the airbag to expand.
44. One Tire, One Molecule
Tires are giant pieces of rubber, so they seem like they must be made of many different molecules. However, rubber tires are actually made up of one massive chain link of polymers, making it just one, singular molecule.
43. Snow to Water
The old legend of one inch of water is equal to 10 inches of snow is true—but not always. This ratio is true for when it is snowing at 30 degrees Fahrenheit, but the ratio increases as the temperature drops, and decreases with a rise in temperature. For instance, when it is 20 degrees, the ratio is 20 inches of snow to one inch of rain, and for 40 degrees, it is of 5:1.
42. Magical Expansion
Water is special, and we all know that, even if you cut class as a kid. When water freezes it expands, obviously, but if you think about it, it shouldn’t be so obvious. Typically, materials consolidate and shrink as temperatures drop, but water and its unique structure have a lot of space for energy to be released, resulting in its expansion.
41. Frozen
Interestingly enough, water also freezes faster when it is warm, and not cold.
40. Can You Smell It?
There’s nothing like the fresh, clean smell after a good thunderstorm. This is due to the chemical reaction lightning has on the earth when it strikes. When lightning strikes, it creates ozone—yes, that ozone, the oxygen compound which protects the earth—by fracturing oxygen molecules, which then reform into ozone, and release a smell into the air that lingers.
39. Water Babies
Adult humans are typically made up of approximately 60% water, however, at birth, we consist of nearly 80% water. After one year, the water content drops to 65%, and as the child ages, it stabilizes at 60%.
38. Do Not Try This At Home
Let’s say you have a room with wooden walls and it is heated to 68 degrees Fahrenheit. You are also using an electric drill to drill through the wall, in an attempt to light the house on fire. No problem. Your power drill is using 750 watts of electricity, on average, and it takes 2,000 joules of energy to heat up a kilogram of wood, so it would take only four minutes of constant drilling to achieve the desired effect. Voila! You can collect your insurance money.

History's most fascinating stories and darkest secrets, delivered to your inbox daily.
37. Natural Heating
Or, how about you don’t light things on fire. Instead, just heat your house up with people! It’s easy, as humans radiate around 150 watts on average, it would take only 70 people to warm up your place in the winter, as long as you keep them in constant motion. Don’t have 70 people? Well, what else is Facebook for? Get that event invite created!
36. Copper Filter
The only metal which is also naturally antibacterial is copper. So, yes, there is some science behind the copper water bottle that your friend carries everywhere.
35. Daddy
The “father” of the periodic table is Dmitri Mendeleev. He created the periodic table of elements essentially as a giant cheat sheet. Working as a professor at St. Petersburg State University, he had to submit a description for all chemical elements but was pressed for time, so he simply slapped together a large data set of atomic weights. This is why the table is ordered by atomic weight.
34. Controversial Chemist
Mendeleev was a controversial figure during his lifetime. His second marriage caused a public controversy because his divorce was not yet finalized, and in Imperial Russia, one had to be divorced for seven years before they could remarry, making his marriage illegal. Due to this, the Russian Academy of Science refused him from being accepted, even though he was responsible for the transformation of Russian chemistry.
33. Let It Grow
Shaving sucks. We all know it, but not many of us have the courage to just say screw it, I am growing my whole body out. But Dmitri Mendeleev didn’t care and was known to only trim his beard once a year, each spring. Upon being called in to see the Tsar of Russia, he still didn’t even bother to groom himself.
32. Chemists Law
Mendeleev created the Periodic Law, in which he described the underlying pattern of all 63 known elements from his time. He used this to create a table, which then anticipated new elements by having blank spaces, creating a new race to discover what elements were missing from the bank of human knowledge.
31. Ego in the Way
Mendeleev than used this empty spaces to anticipate not-yet-discovered elements, and even to predict what their weights and chemical behaviors would be. However, this also led him to deny the existence of elements he did not foresee, as he did with the discovery of argon, gallium, scandium, and germanium, simply because they didn’t fit in his table.
30. The Chum Bucket
Chalk is an essential part of childhood, from school to playing on the sidewalk, and surely you’ve tasted it at some point. It wasn’t very good, was it? It's pretty damn dry, and this may have something to do with its makeup: it’s made of trillions of fossilized plankton. Maybe it’s a part of Sheldon J. Plankton’s plan to steal the Krabby Patty recipe.
29. Fight Fire With Sperm
As a natural flame retardant, DNA is now being used to create flame retardant fibers. The investigation into how to replace commercial flame retardants with this naturally recurring, environmentally friendly structure that makes up all life on earth is ongoing.
28. Semen Shirts
One of the ways in which DNA is being experimented with is by covering cotton cloths with the DNA extracted from the sperm of herring fish. Mmm, how appealing.
27. Thanks, Saliva
Food is a necessity, however, food that tastes good is one of the greatest pleasures of life. You can thank your saliva for this, as without that moisture, you wouldn’t be able to taste anything. Saliva works to break down the foods, which in turn dissolves the chemicals onto the tongue’s taste receptors.
26. How Light Can You Go
The lightest element consisting of solely radioactive isotopes in Technetium, which is also the first artificially made element. However, technetium does not even have one stable isotope. As it was the first mostly artificial element to be added to the periodic table, its name is derived from the Greek word for “artificial.”
25. Heavy Does It Get
The heaviest element in the world is Oganesson. First conceived of in 1979 and given the placeholder name of Ununoctium, this synthetic element was finally developed and recognized in 2016. Upon its synthesis, it was renamed after the important nuclear physicist Yuri Oganessian.
24. No, We Were First
Oganessian is one of only two periodical elements to be named after a living person, the other being Seaborgium, which is another synthetic element, known for being the most stable isotope. Atoms of this element were found by both Soviet and American scientists in 1974, which led to a dispute that would last 23 years between the two countries.
23. Metal Table
Metal makes up the most widespread type of element on the periodic table. Interestingly enough, the only two non-silvery metals are gold and copper.
22. Filling the Balloon
Helium has the ability to not just make your voice high, but can also get itself high. Being lighter than oxygen and the air around us gives it the ability to float.
21. Origins of Our Origins
Though carbon is everywhere, even inside of stars, it was not produced by the Big Bang. Instead, carbon atoms are produced by helium atoms which are fused together during the formation of red giants by dying stars.
20. Forming a Giant
Red giants get their color, or at least the color perceived by the human eye, during the process of cooling down and expanding when all of their hydrogen is finally used up. This will happen to our own sun in about 5 billion years, and it will swallow up Mercury and Venus in the process.
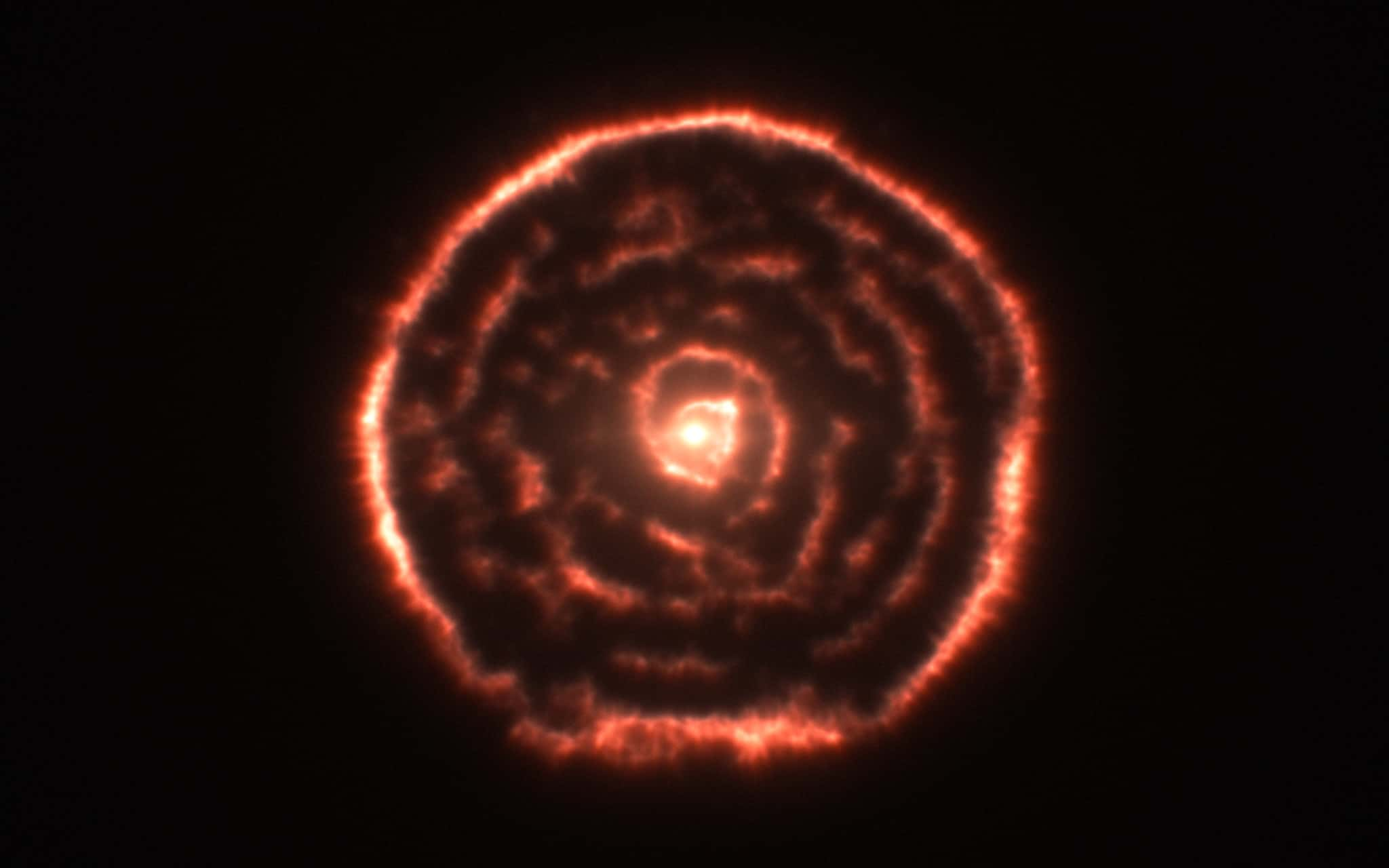 Flickr, European Southern Observatory
Flickr, European Southern Observatory
19. Diamond Tears
The word carbon was comes from the French word for coal—charbon. It was named in the 18th century after Antoine Lavoisier figured out that a diamond consisted of carbon. He did this by building a tool to focus sun rays (a solar furnace) and then used it to burn a diamond and analyze the residue. It wasn’t until then that people realized that carbon wasn’t just coal, but a unique chemical substance.
18. Human Makeup
There are 94 naturally occurring chemical elements in the world, and our bodies are made up over 60 of them. These are essential to our makeup, but only six of them make up for 99% of us: oxygen, carbon, hydrogen, nitrogen, calcium, and phosphorus.
17. Icy Hot
We refer to diamonds as “ice” because of their look, right? Wrong. Actually, “ice” isn’t just slang, but has a real logic behind it. Diamonds have the ability to transport heat, which in turn makes them icy to our touch. There unique thermal conductivity is due to the three-dimensional weaving lattice structure of their makeup, which turns heat into easily transportable lattice vibrations.
16. Carbon as Blanket
Just because something becomes a buzzword and is used as political rhetoric doesn’t always mean you have to be skeptical of it. The chemical science behind the heating of our planet and its subsequent climate change is relatively simple: carbon dioxide cohesively surrounds planet earth, preventing too much heat from escaping, and thus creates the warm conditions that sustain life. As humans burn more and more fossil fuels, more carbon dioxide is released into the atmosphere, and more heat is trapped on the planet. There, explain it that way to your uncle at Thanksgiving and maybe he’ll get it this time.
15. Special Vacuum
In order to boil, water needs a special environment of pressure, but not necessarily very high temperatures. In fact, though space is incredibly cold, water boils when exposed in space. The first thing that happens upon exposure to the vacuum of space is a rapid boiling, followed by an instant freezing.
14. Pissing in the Space
How did we figure out the odd behavior that water exhibits in space? The most natural way, of course: by peeing. Astronauts noticed that when they relieved themselves in space conditions, the pee violently boiled, then went through sublimation as its vapor immediately turned solid. The result was a cloud of frozen pee floating in space.
13. Talc is Everywhere
Everyone knows talcum powder—it's ubiquitous in modern society. This is because talc is the softest known chemical substance, making it perfect for everything from drying basketball players hands to lubricating things.
12. Dried Up
Dry ice is...well, actually what is dry ice? It is carbon dioxide in its solid state. That’s what it is.
11. Liquid Swords
Though metal is, well a solid, not every single metal is solid at room temperature. Mercury is the lone metal that actually takes on another form at normal conditions, all the way down to 0 degrees Celsius.
10. Oxygen Colors
Oxygen is clear to the human eye, however, when it passes into a liquid state, it takes on the color blue.
9. Froggy Skin
Human skin has proteins in it which waterproof us in order to keep water from escaping our bodies, which also limits our absorption of outside water. The frog, on the other hand, doesn’t need to even drink water, as its skin absorbs water out of the air.
8. Ripen Machines
Have you ever noticed that when apples start to ripen, other fruits and vegetables around it also begin to ripen? No, I did not just make that up—pay better attention to your fruit bowl! Well, this is because as apples ripen, they release the gas ethylene, which works to ripen many different types of fresh produce. So, for those of us in colder climates, you can keep your apples next to the hard green lumps that grocery stores call avocados when you unpack your groceries.
7. Rust Life
Mars looks red to us due to its abundance of iron oxide. Another word for it: rust. Mars is a big, ol’ rusty ball floating in space.
6. Shattering Glass
Metals are composed of densely packed atoms, however glass, unlike other solid materials, contains a random assortment of atoms which are relatively free from each other and widely dispersed. Because of this composition, glass cannot absorb energy, as the atoms aren’t close enough to sustain its structure upon energy transfer, which leaves it highly fragile.
5. Inbetweeners
Glass is actually not in a solid state, but rather is in something called an amorphous solid, which means a material which is not solid but not liquid. Well, what does that mean? It means that glass is kind of a liquid which lives its life leisurely flowing along.
4. Basis For Physical Life
Carbon is the basis for all life on earth, as it can bond with many other chemical elements, but it can also bind with itself and subsequently comes in many different physical forms, including both diamonds and graphite—some of the hardest and the softest substances of the earth.
3. How Salty
Archimedes’ Law set the foundation for the fact that when you enter into a bath, the water level immediately rises. Yet, when you add salt itself into the water, the level of salt in the water actually decreases, as the dissolving ions in the water allow the solvent molecules to order themselves better.
2. Cutting Calories
In the same vein, if you were to add half of a liter of alcohol into half of a liter of water, you would end up with less than one liter of total liquid. Just another example of the wonder of alcohol. You’re welcome.
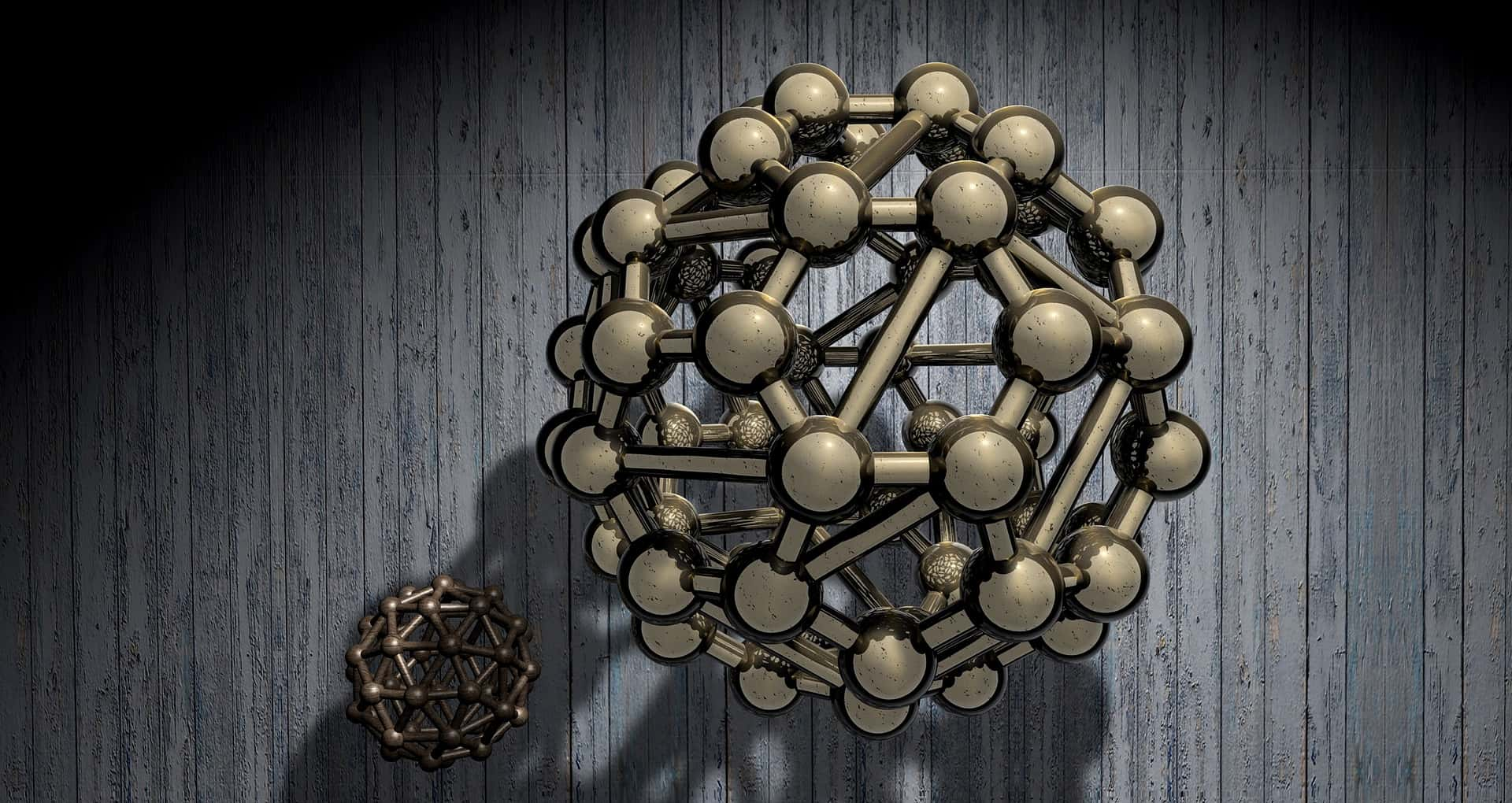
1. Too Rich for My Blood
A startup company out of Oxford University has developed a type of molecule called an endohedral fullerene, also known a "buckyball." These molecules sell for the equivalent of $167 million per gram. The only more expensive substance? That would be the mythical stuff known as antimatter. Scientists have actually managed to produce some of the stuff, but an entire gram of antihydrogen would seriously cost $62.5 trillion.
Sources: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23